What is Green Chemistry?
Green Chemistry is a relatively new emerging field that strives to work at the molecular level to achieve sustainability. Starting in the mid-1990s, this has developed as a dynamic and rapidly developing discipline.
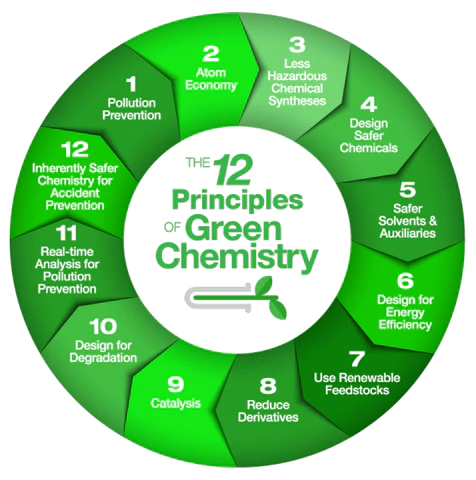
The Twelve Principles
Green Chemistry has a framework of a cohesive set of Twelve Principles, which have been systematically surveyed, to meet environmental and economic goals simultaneously.
These Twelve Principles were introduced by Paul Anastas and John Warner. This is a guiding framework for the design of new chemical products and processes, applying to all the chemical aspects from the raw materials used in enhancing efficiency and safety of the transformation, to the toxicity and biodegradability of products and reagents used.
The Concept of Design
Design is the most important aspect of Green Chemistry. This is a statement of human intention and one cannot do design by accident. It includes novelty, planning and systematic conception. The Twelve Principles of Green Chemistry are “design rules” to help chemists achieve the intentional goal of sustainability.
Changing the Bad Old Ways
Environmental Chemistry is largely involved with the problems caused by the improper discharge of pollutants. In recognition of the environmental effects of the chemical industry and the related enterprises, many laws have been implemented throughout the world to regulate chemical processes and products. These laws have unquestionably had very positive effects upon improving environmental quality, as an overall.
However, still there were some limitations in regulating such as poor cost effectiveness, and in worst cases, counterproductivity.
Industrial Ecology
As an alternative to the regulatory approach in preventing pollution, the practice of Industrial Ecology was introduced. Green Chemistry and Industrial Ecology are strongly connected and one cannot be practiced effectively without the other. There are numerous examples of successful industrial changes that used Green Chemistry.
As the first example, a greener synthetic pathway was attributed to Eastman for its enzymatic esterification. This biocatalytic process runs under mild conditions, minimizes the formation of byproducts and saves energy, resulting in an increased efficiency. Overall hundreds of litres of organic solvents were eliminated from the previous process.
In 2008, researchers were rewarded for the design of green pesticides. While trying to understand the structure–activity relationships of natural biopesticides that would be more active, they designed Spinetoram. It is predicted that this new pesticide will eliminate about 1.8 million pounds of organophosphate insecticides, during its first five years of use.
In 2004, a new approach to Paclitaxel, the active ingredient in the anticancer drug ‘Taxol’ was developed. Paclitaxel is commercially produced from the naturally occurring precursor through a multistep synthesis. This “semisynthetic route”, which was first developed as an economically viable approach to the molecule, was not without certain environmental concerns. A more sustainable process was therefore investigated by using the latest biotechnological advances. Instead of synthesizing Paclitaxel from a precursor, the active compound was extracted directly from plant cell cultures.
Conclusion
For generations, molecular scientists have invented the molecules, materials, and manufacturing processes that have allowed economic and societal development. Green Chemistry is ensuring that all of that creative ability is practiced in a way that builds in impact on people and the planet as a design criterion. An impressive amount of work has been done by practitioners of Green Chemistry around the world, and those achievements remark the power and potential of the field. In the future, more interesting applications will be designed, using the cutting edges of technology.
Written By: Mokshana Alahakoon
References:
- Anastas, P. and Eghbali, N., 2010. Green chemistry: principles and practice. Chemical Society Reviews, 39(1), pp.301-312.
- Manhan, S. Environmental Chemistry – 10th edition
Image Courtesy:
Cover Image – http://bit.ly/3NmqMku
Image 1 – https://bit.ly/3uVBrfR
Image 2 – https://bit.ly/41eS1Dk