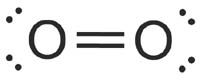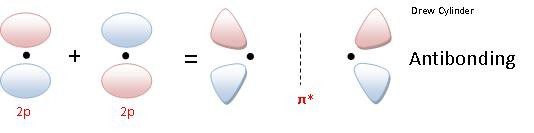Molecular Orbital Theory…… It’s a simple theory to be understood. Initially we draw Lewsi structures for molecules. And we derived the shapes of molecules using the VSEPR theory.
But by these we failed in explaining some facts about bond formation. As a result of this Quantum Mechanical Theories came up to explain about the formation of bonds. The well-known theories are “Valence Bond Theory” and “Molecular Orbital Theory”.
So it’s understood new theories came up due the drawbacks in the existing theories. The drawback in the Lewis Theory is that it cannot be used to explain the fact that why chemical bonds exist. And according to the Lewis Theory the H-H bond in the H2 molecule is more similar to the F-F bond in the F2 molecule. But the bond enthalpy values if these two molecules are different.
So for a better and a complete explanation of the chemical bonds that are formed we looked into the Quantum mechanics and two theories came up.
Frist one: its Valence Bond Theory…. Here it simply explains how a bond is formed…. Let us take a simple example…Hydrogen molecule…. H-H bond is formed by the overlap of the 1s orbitals of the H atoms where as in Fluorine it’s the overlap of two P orbitals. After the overlap of the atomic orbitals the electrons that were in the atomic orbitals share a common region and the electrons will be localized to this shared region. Therefore Valence Bond Theory is known as Localized Theory. Valence Bond Theory also gave the basis for the concepts like Resonance, Hybridization, etc…
“Valence Bond Theory” it’s a very simple explanation for that. To understand Molecular Orbital Theory it is very important to see how it developed from these existing theories for bond formation.
The problem that came up with the Valence Bond theory is that it cannot be used to explain some characteristics of some molecules. According to the VBT all the electrons are paired and they reside on the shared region of atomic orbitals. But each bonding electron is characteristic for the whole molecule but not for a specified region. If the bond of O2 molecule is considered all the electrons are paired.
Image courtesy: http://www.amazingrust.com
Then O2 has to be diamagnetic. But it’s Paramagnetic. Now you must be wandering what this Diamagnetic and paramagnetic means 😀
Diamagnetic means: Molecules are repelled by an externally applied magnetic field which means the molecule doesn’t have unpaired electrons.
Paramagnetic Means: Molecules are attracted by an externally applied magnetic field. For a molecule to be paramagnetic it has to have unpaired electrons.
So to explain these confusions….Here comes the “Molecular Orbital Theory”……
The MBT (Molecular Orbital Theory) explains the formation of Covalent Bonds in terms of Molecular Orbitals and explain that molecular Orbitals are formed by the combination of atomic orbitals and these formed molecular orbitals are not for one single atom. It is for the whole molecule.
Before explaining this further you guys need to understand the meaning of one important word. i.e. “wave function”
A wave function is a function that describes the probability of a particle’s quantum state as a function of position, momentum, time and spin.
Understood it???? 😀 okay I will explain. We all know that an electron has both a particle and a wave nature. An atomic orbital is the space in which the probability of finding an electron is maximum. The volume of this 3D space was calculated by mathematicians using the wave nature of electrons and explained the shapes of atomic orbitals. The wave function of an atomic orbital simply describes the basis in which an electron can reside.
Molecular Orbitals are formed by the combination of these atomic orbitals. Therefore the wavefunction of the molecular orbital is the addition of the wavefunctions of the atomic orbitals that participated for the formation of it.
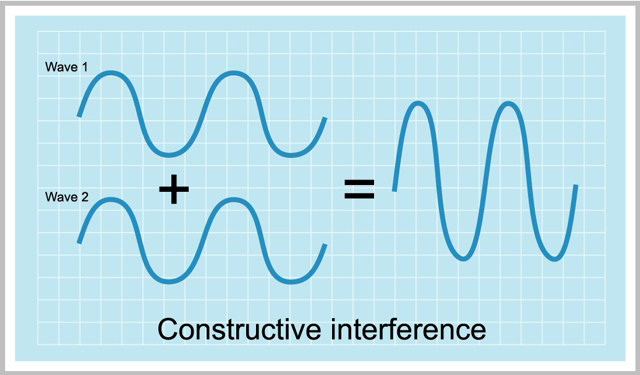
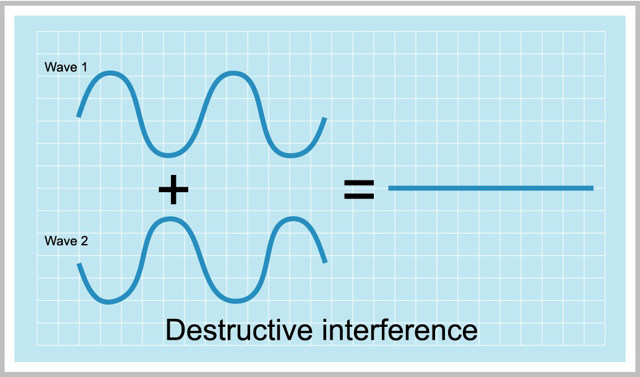
A wave function can have either + or – values. So there are can be two ways of combining of atomic orbitals.
- both the wavefucntions are either positive or negative
- one is positive and one is negative
This gives two types of results.
The first one it’s a “CONSTRUCTIVE INTERFERENCE” i.e. an in phase overlap.
The second one it’s a “DESTRUCTIVE INTERFERENCE” i.e. an out of phase overlap.
Image courtesy : http://astro-canada.ca/
These two interferences make two different types of molecular orbitals.
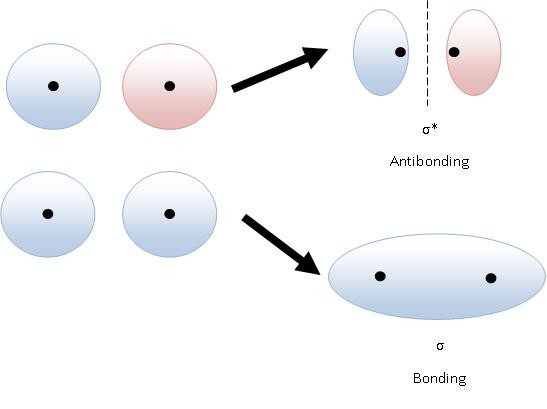
v Constructive Interference – It’s a bonding molecular orbital.
v Destructive Interference – It’s an anti-bonding molecular orbital.
So now you see that if two atomic orbitals overlap two molecular orbitals are formed.
Overlapping of atomic orbitals it can happen in two ways. Either it can be a linear overlap or a lateral overlap.
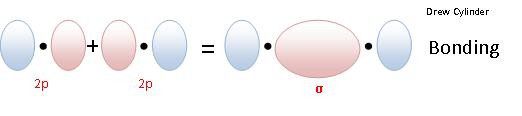
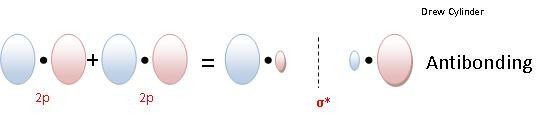
LINEAR OVERLAP Results a Sigma molecular orbital.
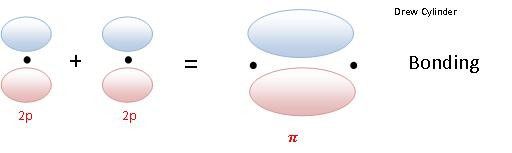
LATERAL OVERLAP Results a Pi molecular orbital.
Sigma Bond: The electron density is concentrated around symmetrically around the line between the two nuclei of the bonding atoms.
Pi bond: The electron density is concentrated above and below the plane if the nuclei of the bonding atoms.
I think now you understand the type of interference and the type of the overlap will result 4 kinds f molecular orbitals.
- Sigma Bonding Molecular Orbital (σ)
- Sigma Anti-binding Molecular Orbital (σ*)
- Pi bonding Molecular Orbital (π)
- Pi Anti-Bonding Molecular Orbital (π*)
So if two 1S orbitals overlap linearly to form a sigma bonding orbital the notation to denote that molecular orbital is: σ1s
So if two 1S orbitals overlap linearly to form a sigma anti- bonding orbital the notation to denote that molecular orbital is : σ*1s
So if two 2pz orbitals overlap linearly to form a sigma bonding orbital the notation to denote that molecular orbital is : σ2pz
So if two 2pz orbitals overlap linearly to form a sigma anti-bonding orbital the notation to denote that molecular orbital is : σ*2pz
So if two 2px orbitals overlap laterally to form a sigma bonding orbital the notation to denote that molecular orbital is: π2px
So if two 2px orbitals overlap laterally to form a sigma bonding orbital the notation to denote that molecular orbital is: π*2px
Image courtesy : http://chemwiki.ucdavis.edu/
Hope that you haven’t got bored in reading this up…. 😀
Then comes the diagrammatic representation of this. “Molecular Orbital Energy level Diagram”
Even it’s not rocket science. It’s too much for the day…
Wait for the next article to learn about it….. 😀
References:
- http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/Molecular_Orbital_Theory
- http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/mo.html
- Chemistry 9th edition by Raymond Chang
Image – http://www.altavetta.com