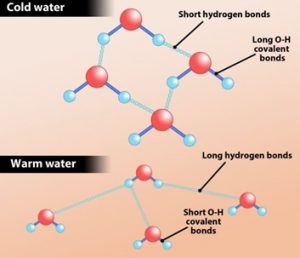
When logically thought, one would think cold water freezes faster than warm water but experimentally it has been found that this could be the total opposite. This phenomenon is known as the Mpemba effect and it is due to the chemical bonds which bind the neighboring water molecules together.
This effect was first observed by Aristotle in the 4th century BC. Later in 1960s, a Tanzanian student named Erasto Mpemba noticed this effect which was named after him as the Mpemba effect. While making ice cream one student placed his mixture of milk and sugar in the freezer without boiling. Mpemba, worried that he would not have space in the freezer immediately put his boiled mixture into the freezer without allowing it to cool down. Both pupils had returned one and a half hours later to see Mpemba’s mixture had frozen and the other had not. Mpemba did not brush his curious observations aside but instead asked his teachers, friends and even his ice cream selling friends to explain the reason behind his observations. Mpemba had finally asked a visiting lecturer from the University of Dar es Salaam to explain the reason behind his observations. The unprejudiced Dr Osborne was intrigued by Mpemba’s observations and began investigating the effect with his students. He published a paper with Mpemba on the observed effect in 1969.
Although the effect was found, many scientists carried out experiments to prove the validity of the phenomenon. Scientists have provided various explanations to the phenomenon including evaporation, convection currents, dissolved gases or impurities present in water, but none of these explanations have been fully accepted.
A paper was published online on December 6th in Journal of Chemical Theory and Computation to explain this effect. Professor Dieter Cremer of Southern Methodist University in Dallas and colleagues who carried out experiments on this suggested that the Hydrogen bonds linking neighboring water molecules could be the reason behind it. “We see that hydrogen bonds change when warming up water. The strength of hydrogen bonds depends on the arrangements of nearby water molecules. In simulations of cold water, both weak and strong Hydrogen bonds were observed, but in higher temperature simulations, a larger percentage of the Hydrogen bonds were strong, because the weaker ones were broken to a large extent,” as mentioned by Cremer. Water when heated causes the weaker bonds to dissociate resulting a group of molecules to form fragments that realign to form crystalline structure of ice. This may have been the starting point of the freezing process. For cold water to freeze, first the weaker bonds have to be broken. Although the effect that caused the phenomenon was noted, chemist William Goddard of Caltech pointed out that they had not stimulated the freezing process and shown that it proceeds faster when the new hydrogen bonding insights were included.
Despite many controversies and effort by many scientists over thousands of years, a team of researchers at Singapore’s Nanyang Technological University believes they have solved the mystery of why warm water freezes faster than cooler water under certain conditions recently. They believe it is the way energy stored in stretched Hydrogen bonds that is behind this magnificent phenomenon. The paper has been uploaded to the preprint server.
A water molecule is made of two Hydrogen atoms and one Oxygen atom linked by covalent bonds. Hydrogen atom is linked to an Oxygen atom of a neighboring molecule through hydrogen bonds. But, at the same time, the water molecule is repelled from each other by a repellent force. The research team in Singapore has observed the distance between the neighboring water molecules to be greater in warm water than in cool water due to the repellent force. As a result, it causes the Hydrogen bonds to stretch out more and store more energy. The stored energy is released as the water cools down allowing the molecules to come closer to one another. Since warm water has more Hydrogen bonds stretching than cool water, it stores more energy and thus releases more energy when exposed to freezing temperatures. Therefore, the researchers have solved the riddle that, warm water freezes faster than cool water.
So far what has been stated by the research team seems to be logical and is still a theory until they or others find a way to prove what they have suggested is true by continuous experimentation. This must be done sooner before the scientific community deem the mystery of warm water freezing faster, solved once and for all.
Reference: http://www.nature.com/articles/srep37665
Image courtesy:
http://csl.dynamicpatterns.com/wp-content/uploads/2013/05/375869486_1361564020.jpg
http://i.dailymail.co.uk/i/pix/2013/11/01/article-2483383-192104D300000578-349_634x570.jpg
https://mohdabubakr.files.wordpress.com/2010/05/image5b55d.png
http://image.wikifoundry.com/image/3/627d913dd290fc797dee3dfa28cf4594/GW401H264
http://chargedmagazine.org/wp-content/uploads/2013/06/1-300×225.jpg