The optical microscope was a great discovery which enabled us to get a better view of the cellular scale. However, the resolution is fundamentally limited as a result of diffraction also known as the Abbe’s limit. The Nobel Prize in Chemistry 2014 was awarded for the “development of super-resolved fluorescence microscopy”, a technique which circumvented the above limitation. Three research groups in USA in year 2006 achieved super resolution based on single molecule fluorescence localization while, a group in Germany achieved the same based upon a point spread function re-engineering . They were able to get more accurate images of living cells as a result of the development of ultra-high-resolution microscopy methods. I got an opportunity to interview Dr. Siyath Gunawardene PhD (Physics), Senior Lecturer at Department of Physics, who was a participant in Localization based super-resolution imaging in one of the research teams at University of Maine, USA to get a glimpse of the experiment “Ultra-High Resolution Imaging of Biomolecules”, as bellow;
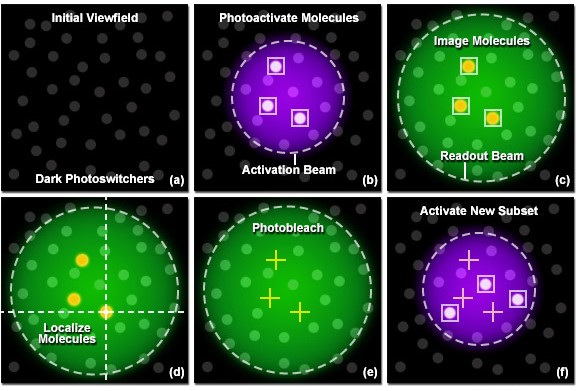
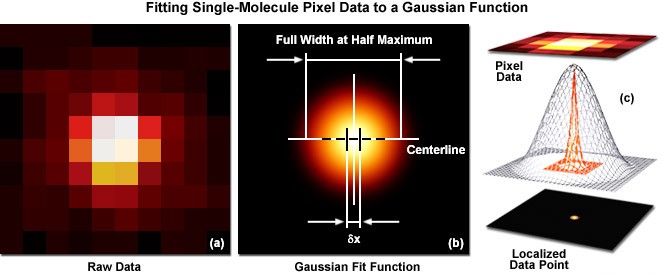
The ultra-high-resolution microscopy technique uses specially modified probes which are known as “photoactivatable fluorescent probes” which can be attached to a protein of interest in a sample. First a sample is selected from a prepared “cell culture” and the probes are then attached to the required proteins. This process is known as tagging. Initially all the molecules (tags) are in an inactive state. By using a low intensity activation Beam, a small number of molecules (tags) are activated as illustrated above (figure 1b). Then a Green laser beam is used to readout the activated molecules. These fluorescent molecules spontaneously photobleach (i.e. attain an irreversible state which cannot be reactivated using the above activation beam) significantly reducing the number of visible molecules. A new set of molecules is activated again by briefly turning on the activation beam. A high sensitivity camera is used to record the whole process. This process is repeated many times until enough molecules have been activated and imaged to obtain the desired image quality. These frames are analyzed to identify and localize activated molecules. Precise localization is done by fitting a Gaussian to the intensity pattern of each point. (Figure 2)
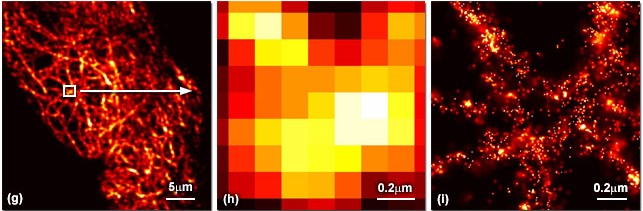
Fluorescence photoactivation localization microscopy (FPALM) method can image living or fixed cells with measured resolution better than 20 nm which is 10 times greater than the light microscope. Researchers are now able to get 3D images using this same technique by considering multiple planes. FPALM can be performed on a variety of biological samples which will significantly advance the understanding of biological systems.
References
http://zeiss-campus.magnet.fsu.edu
http://www.nobelprize.org/